Basic ChemTeX codes
Updated 17 Sep 2025
ChemTeX is a subset of TeX. Use the following ChemTeX codes when editing or creating chemical formula or expression answers. While some variations on the codes below may work, use these basic codes to optimize the performance of your content.
- Sample ChemTeX answer strings with rendered forms
- To view the rendered chemical expression as you edit: Select the Preview link. Entering ChemTex in other text boxes will not show the rendered ChemTex. About entering ChemTeX in the Simple Editor
| Answer attribute | Examples | ChemTeX code |
|---|---|---|
| Dot | \cdot | |
| Fraction | \frac {top}{bottom} | |
| Reaction symbols | ||
| Forward reaction | \rightarrow | |
| Equilibrium reaction |
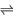
|
\rightleftharpoons |
| Stacked super/subscript isotope notation |
|
{^{super}_{sub}} or {_{sub}^{super}} Single-digit super/subscripts do not need to be enclosed in a set of {}. |
| Subscript |
|
_{sub} or _s
Single-digit subscripts do not need to be enclosed in a set of {}. |
| Superscripts | ||
| Pre-superscript only (isotope notation) |
|
{^{super}} or {^s}
The entire superscript expression must be enclosed in {}. Single-digit superscripts do not need to be enclosed in an extra set of {}. |
| Ion notation |
Single atoms, like: Molecules, like: |
{^{super}} or {^s}
The entire superscript expression must be enclosed in {}. Single-digit superscripts do not need to be enclosed in an extra set of {}. |
|
Molecules ending with a subscript, like:
- 2- |
{molecule ending with a subscript}{^{super}}
A molecular formula that ends with multiple atoms of an element must be enclosed in {} before attaching the charge as a post-superscript. |
|
| Symbols and variables | ||
| Antineutrino | e and | \bar{\nu}_{e} or \bar{\nu} |
| Energy | energy, Energy, ENERGY | energy, Energy, ENERGY (not case- sensitive) |
| Equations of state | (s), (l), (aq), and (g) |
(s), (l), (aq), and (g)
s = solid, l ("ell") = liquid, aq = aqueous, and g = gas |
| Excited nuclei | * * |
{^\ast} or {^*} |
| Heat | heat, Heat, HEAT | heat, Heat, HEAT (not case-sensitive) |
| Lowercase Greek | \alpha, \beta, \gamma, and \nu | |
| No reaction | noreaction, Noreaction, NOREACTION |
noreaction, Noreaction, NOREACTION (not case-sensitive)
Use for questions that ask students to predict a product formed when there is no reaction. When no reaction is used, it must be the complete answer. |
| Placeholders | Elements: |
A, B, C, D, X, Y, and Z |
| Lone pair of electrons: | E | |
| General ligand: | L | |
| Generic metal: | M | |
| Arbitrary metal or metal ion: |
Me, Me{^+} or Me{^-} |
|
| Organic functional group: | R | |
| Arbitrary radical: | R | |
See also: