Mechanism Rules evaluator to grade chemistry answers
Updated 17 Sep 2025
Use the Mechanism Rules answer evaluator (expressionOchemEvalMechanismRules) to check whether a particular heuristic rule is or is not violated by the student response in a mechanism question. When this expression is triggered, the step box that contains an error changes to red.
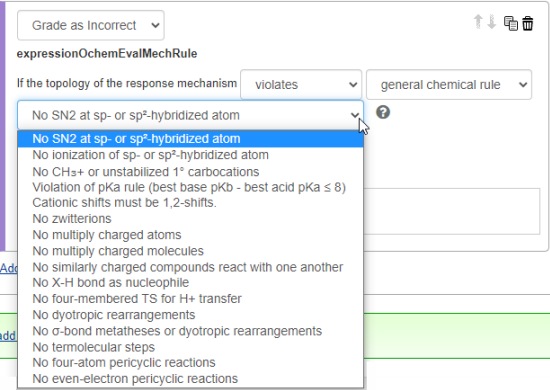
- Select Grade as Correct or Grade as Incorrect, then enter the conditions you want to be checked in student answers.
- Choose violates or does not violate.
- Select the rule category you want evaluated.
- general chemical rule
- reaction-condition-specific rule
- mechanism drawing convention
- Select the specific rule from the category.
 General chemical rules
General chemical rulesMenu option Description No SN2 at sp- or sp2- hybridized atom Does the student answer include curved arrows from a nucleophile to an atom incompatible with an SN2 mechanism (SN2 reactions must occur at sp3-hybridized atoms)? No ionization of sp- or sp2- hybridized atom Does the student answer result in a product that places a charge on an sp- or sp2-hybridized atom? No CH3+ or unstabilized 1° carbocations Does the student answer result in a product that places a charge on a primary carbon atom? Violation of pKa rule (best base pKb – best acid pKa ≤ 8) Does the student answer contain unbalanced acids and bases?
Select a checkbox to ignore the first or last step of the mechanism.
- Check here to omit the first step of the mechanism (e.g., because a strong base initiates the reaction).
- Check here to omit the last step of the mechanism (e.g., because it is a workup step).
Cationic shifts must be 1,2-shifts Does cation rearrangement contain shifts other than 1,2-shifts? No zwitterions Does the student answer include a zwitterion (such as an amino acid)? No multiply charged atoms Does the student answer include an atom with a charge greater than +1 or less than -1? No multiply charged molecules Does the sum of the charges on the atoms in a molecule result in a total charge greater than +1 or less than -1? No similarly charged compounds react with one another Do two molecules with the same charge (i.e., both positive) react with one another?
Select the checkbox to allow proton (H+) transfer steps such as:
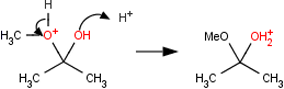
No X–H bond as nucleophile Does the student indicate that an X-H bond, where X is a halogen, acts as a nucleophile? For example:
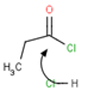
No four-membered TS (transition states) for H+ transfer Does the student indicate that a step involves a four-membered transition state during a deprotonation? For example:
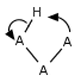
No dyotropic rearrangements (A–B–C–D → D–B–C–A) Does the student indicate a step that involves multiple rearrangements in the connectivity of atoms in a single molecule? For example:
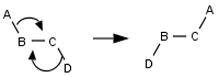
No σ-bond metatheses or dyotropic rearrangements Does the student indicate that a step involves multiple rearrangements in the connectivity of atoms in multiple molecules or a single molecule?
For example:
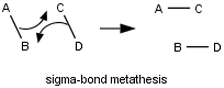
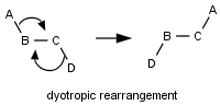
No termolecular steps Does the student answer contain a step requiring three molecules to come together? No four-atom pericyclic reactions Does the student answer contain a transition state that has a cyclic geometry and only four atoms? No even-electron pericyclic reactions Does the student answer contain a pericyclic reactions (other than electrocyclic reactions) that involves an even number of pairs of electrons?  Reaction-condition-specific rules
Reaction-condition-specific rulesMenu option Description No radicals under polar conditions Does the student answer contain a radical under conditions that do not support a radical? No carbocations (except iminium ions) under basic conditions Does the student answer contain a radical under conditions that do not support a radical? No bases under these acidic conditions should have pKb > Does the student answer contain a base, explicit or implied, with pKb greater than that specified?
Enter a pKb value.
(The student response may show the bases explicitly or may produce them in a mechanistic step but omit them from the drawing.)
No acids under these basic conditions should have pKa < Does the student answer contain an acid, explicit or implied, with pKa less than that specified?
Enter a pKa value.
(The student response may show the acids explicitly or may produce them in a mechanistic step but omit them from the drawing.)
No SN2 under acidic conditions. Does an SN2 reaction occur in the absence of a strong base? No E2 under acidic conditions. Does an E2 reaction occur in the absence of a strong base?  Mechanism drawing convention rules
Mechanism drawing convention rulesMenu option Description No atom should receive & supply electrons Does the student answer include curved arrows to and from the same atom? No resonance arrow where there should be one Does the student use a reaction arrow to indicate movement of electrons between two resonance contributors?
Select the checkbox to skip the first step of a photochemical mechanism.
Resonance arrow where there shouldn't be one Does the student use a resonance arrow to indicate an organic reaction?
Select Show this response to enter wrong answer feedback for when the student’s answer matches a Grade as Incorrect condition.
Use your keyboard and options from the editing toolbar and menus. Wrong answer and follow-up text can include an image, link, and formatted text like bullets or TeX.
See also: