Is evaluator to grade chemistry answers
Updated 17 Sep 2025
Use the Is answer evaluator (expressionOchemEvalIs) to compare a student’s drawn molecule to the answer molecule. Both skeletal and mechanism questions support the Is evaluator.
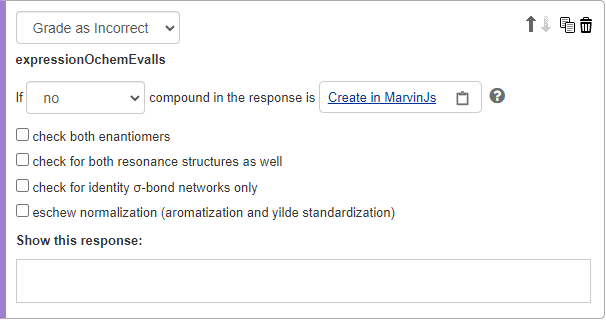
Select Grade as Correct or Grade as Incorrect, then enter the conditions you want to be checked in student answers.
no
None of the molecules in the student’s answer matches the molecule in this evaluator.
the only
The student’s answer includes one and only one molecule which matches the molecule in this evaluator.
exactly one
The student’s answer includes one compound (not more) which matches the molecule in this evaluator. The student’s answer may contain other molecules of different types which do not match the molecule in this evaluator.
any
Any of the molecules in the student’s answer matches the molecule in this evaluator. The student’s response may contain more molecules which also match.
not every
Not all of the molecules in the student’s answer match the molecule in this evaluator.
every
All of the molecules in the student’s answer match the molecule in this evaluator.
Either draw or copy and paste a molecule fragment or substructure. For instructions, see How to add a molecule/compound for comparison.
These options compare the student’s answer to the evaluator answer and any of a set of chemical transformations of the evaluator answer.
- Check both enantiomers
- Check for both resonance structures as well
- Check for identity of sigma-bond networks only
- Eschew normalization (aromatization and ylide standardization)
For example, when “check for both enantiomers” is selected, the evaluator compares the student’s answer to the correct answer and compares the enantiomer of the student’s answer to the correct answer. If either of those comparisons creates a match, the answer is graded as correct
- If you draw a chiral compound without specifying stereochemistry, the system assumes that both enantiomers are included in the evaluation.
 For example
For exampleSay you want to set an answer as being wrong when no stereochemistry is specified. In this case, it’s better to use a Boolean answer evaluator stating that if the specific R enantiomer and the specific S enantiomer are not present, then the answer is wrong.
- The sigma-bond networks refer to the connectivity of the sigma bonds (rather than the pi bonds) of a molecule. For example, choose this option to grade an E and Z isomer as the same.
- Mastering normalizes student responses before evaluating them. Since ylides and aromatics can be drawn in more than resonance form, Mastering normalizes ylides and aromatics to a single conventional resonance form before comparing them.
 For example
For exampleThe structures Me2S+-O- and Me2S="O" are viewed as identical compounds, as are the two aromatic resonance forms of 1,2-dichlorobenzene. The normalization process happens by default.
- When you do not want student answers normalized, use "eschew normalization".
 For example
For example
Choose eschew normalization for "draw a resonance structure" questions. This lets Mastering distinguish different resonance structures of ylides and aromatics. If different resonance structures should NOT be distinguished, do NOT select this option.
Select Show this response to enter wrong answer feedback for when the student’s answer matches a Grade as Incorrect condition.
Use your keyboard and options from the editing toolbar and menus. Wrong answer and follow-up text can include an image, link, and formatted text like bullets or TeX.
See also: